
 |
| Assistant Professor Bethany Buck-Koehntop |
As I edge closer to what looks like a stainless-steel beer fermentation tank, I’m warned by my guide to stay behind a set of hazard cones. “Your credit cards might get erased,” says Bethany Buck-Koehntop, Assistant Professor of Chemistry and large-magnet enthusiast. We’re in a large laboratory space in the “Gauss Haus,” the University of Utah’s David M. Grant NMR (nuclear magnetic resonance) Center.
 |
| The 800 MHz NMR Spectrometer is located in the D.M. Grant NMR Center, also known as the Gaus Haus. |
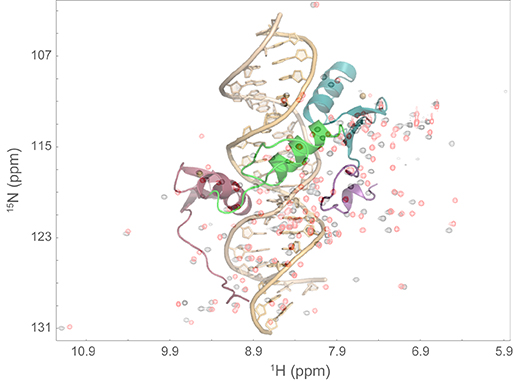 |
| Overlay of a high-resolution 3D protein:DNA structure on a 2D NMR spectrum. |
There’s no beer being brewed here: the tank is the enclosure of one of several impressive superconducting magnets at the facility, which are cryogenically cooled. These magnets are one component of the NMR spectrometers that generate intense magnetic fields, which, in conjunction with selective rf (radio frequency) irradiation, elicit absorption spectra that can be used to probe the structures and dynamics of biomolecules such as proteins and nucleic acids. The resulting experimental data can lead to three-dimensional structures of such molecules, which can in turn reveal atomic-level detail of binding interfaces between macromolecules.
The interactions that interest Buck-Koehntop are those that are involved in the interpretation and translation of epigenetic DNA signals. Epigenetic alterations are reversible surface chemical modifications that control the ways in which DNA is packaged (i.e., the form of its chromatin structure) and therefore the degree to which genes are expressed. The switching on or off of genes partially controlled via methylation of cytosine DNA bases plays an important role in the normal development and functions of cells, and can serve to permanently suppress harmful genes. However, methylation error—the silencing of crucial genes in conjunction with activation of harmful genes—is linked to disease, including cancer.
Buck-Koehntop and her colleagues are studying a set of three methyl-CpG binding proteins (MBPs) known as the ZBTB family, all of which are associated with cancer progression via their regulation of gene expression at methylated DNA sites. The team takes a multidisciplinary approach, combining in vitro biophysical and in cell genomic studies, to identify and characterize the activities of the ZBTB family of MBPs in cancer. The ultimate goals of such studies are identifying new epigenetic-based biomarkers for improved cancer diagnostics, and facilitating the design of cancer therapeutics directed against ZBTB protein regulatory pathways.
Those are long-term prospects, but Buck-Koehntop is optimistic. Here in the Gauss Haus, the optimism feels well founded.
Story by Paul Bernard
