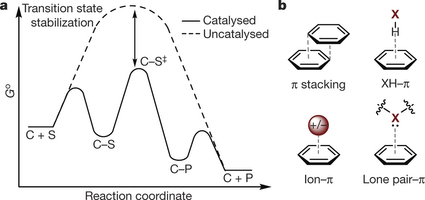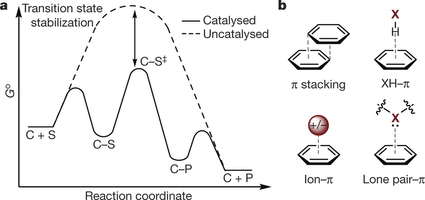
Professor Matthew Sigman and his collaborators have had their recent work with non-covalent interactions featured in an article on Forbes.com. The article references their paper published in Nature, explaining that the paper “takes a close look at one kind of non-covalent interactions, made possible by ring-shaped molecular motifs called aryl groups. They sort these interactions into broad categories based on how the rings are oriented and what other molecular architecture is around them. It’s a first step in building that new library of knowledge chemists would need to be able to effectively engineer catalysts.”