
In 2016 , the Department of Chemistry hosted the inaugural Robert W. Parry Lectureship in Inorganic Chemistry. This annual lecture recognizes Distinguished Professor Bob Parry, one of our most notable former faculty members. The first lecture was delivered by Prof. Harry Gray, a world-class inorganic chemist from CalTech, a friend of Bob Parry’s, and a donor to the fund. Marjorie Parry and other family members are also the atteneeds and supporters of the Parry Lecture.
Distinguished Professor Robert W. Parry was a giant in his profession. He was the founding editor of Inorganic Chemistry in 1960. He served as the President of the American Chemical Society in 1982, won the first Utah Governor’s Medal in Science and Technology in 1987, and received the American Chemical Society’s Priestley Medal in 1993. In his 60-year career, Bob taught thousands of undergrads and mentored over 60 Ph.D. students and postdocs. Together with Henry Eyring and Cheves Walling, Bob played a key role in the growth and development of chemistry at Utah.
 |
 |
The Parry Lecture with Ana de Bettencourt-Dias, University of Nevada, Reno
Tuesday, February 27, 2024, TBBC 4630 (4th floor Thatcher), 10:45AM – 12PM
 |
 |
Abstract: The luminescence of lanthanide ions is best harnessed through appended ligands or sensitizers, in a process called the antenna effect.1-2 While this might seem more complicated than direct f-f excitation, it opens the opportunity to tailor ligands for a variety of applications involving light emission, such as lighting, imaging, and sensing. 3 We have used a building block approach to isolate complexes that can be used as imaging agents for cancer cells.4 By shifting the excitation wavelengths into the visible through extending the conjugated -electron system, we isolated complexes that can be used as molecular nanothermometers, 5 or as in situ reporters of viscosity.6 In addition, functionalizing the ligands with specific functional groups enabled excitation of the resulting complexes in the biological window by a two-photon process.7 The use of oligothiophene-based ligands led to complexes that concurrently luminesce and generate singlet oxygen and show appreciable photocytotoxicity. 8 In this seminar we will discuss the isolation and characterization of several of these complexes and what we have learned from the building block approach to ligand design.
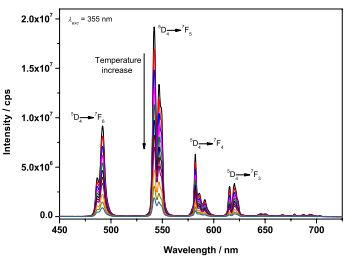
Figure 1. Temperature-dependent emission spectrum of K3[Tb(dipicCbz)3]. Inset shows the intensity of the 5D4 → 7F5 transition as a function of temperature.5
