
Research Spotlight: Professor Janis Louie
 |
|
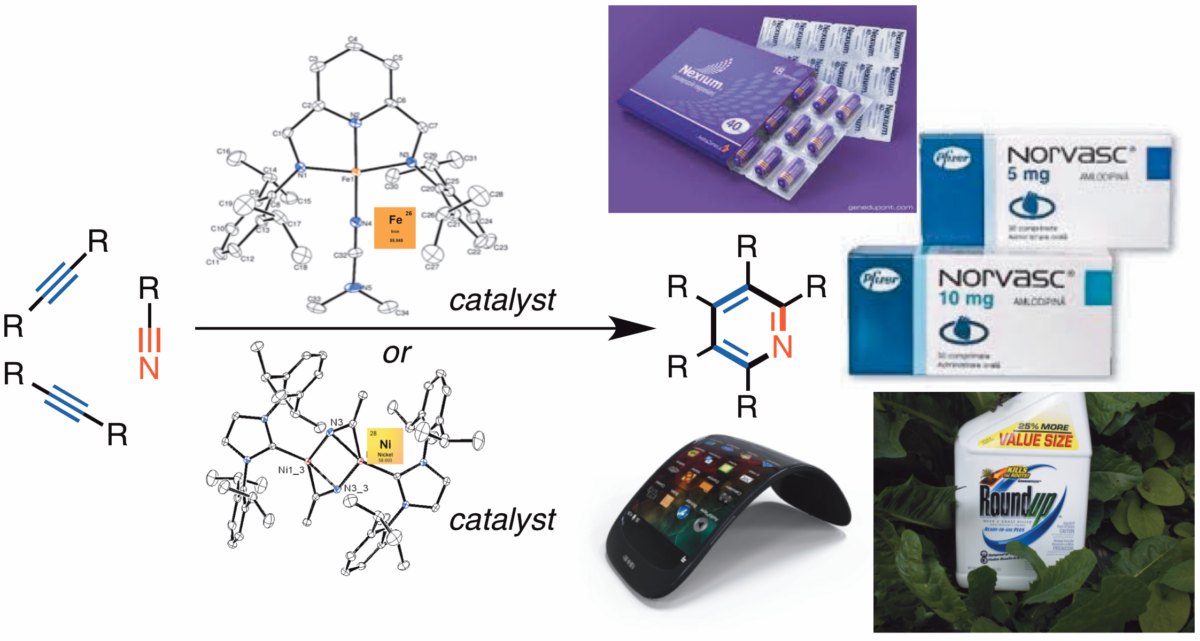
Professor Janis Louie creates new catalysts based on earth abundant metals such as iron and nickel for use in synthetic chemistry. |
Professor Janis Louie always knew that research was for her, before she knew what a career in research even meant.
“I had some idealistic notion that research and development would be just so cool,” Louie remembers. “I had absolutely no idea what research and development meant, zero. I don’t know where that idea came from – no one in my family does research – but somehow it just seemed like a good idea. Luckily for me, I didn’t choose wrong.”
In high school, chemistry was the first class that really challenged Louie. She recalls studying and studying for a midterm, only to throw up her hands, get a good night’s sleep, then ace the test. After that, chemistry really clicked with her, and she started seeing it everywhere.
“Of all the sciences, it’s the best,” she said. “One of my pet peeves is when I hear ‘Oh, I love biology or I love physics or I love math, and I HATE chemistry.’ Chemistry is the whole reason all of this works! I find it so frustrating that chemistry has a bad rap because everything around us is chemistry. If you can touch it, then it’s chemistry.”
Louie took her love of chemistry with her to her undergraduate studies at UCLA. When she took organic chemistry, her decision was solidified.
“If my favorite class is the class that everybody hates, then clearly this is for me,” Louie remembers thinking.
After also enjoying inorganic chemistry, Louie decided to combine the two and study catalysis. She did her graduate work at Yale with Professor John F. Hartwig (now at UC Berkeley), followed by a NIH postdoctoral fellowship with Professor Robert H. Grubbs at Caltech.
Now as a faculty member, Louie’s research group develops new transition metal catalysts that are based on earth abundant metals such as iron and nickel. These catalysts are tools for other synthetic chemists to make heterocycles (a cyclic compound with a heteroatom) in an easier, cheaper, faster, or more efficient way. These heterocycles have uses in pharmaceutical, agricultural, and even electronic industries.
 |
|
Louie’s iron and nickel catalysts create heterocycles that can be used in pharmaceuticals, agricultural chemicals, or electronics. |
Typically, the catalysts that are used most in industry are based on precious metals like palladium, platinum, ruthenium, rhodium, or iridium. Although these precious metals are very expensive, they work very efficiently and as such, constitute almost all catalysts used in fine chemical syntheses.
“My group has taken the approach of trying to coax the more abundant metals, such as iron and nickel, to do more,” Louie explained. “They may not be as efficient, but they’re so abundant that you don’t need them to be as efficient to be more cost effective.” She compares it to making catalysts from rust rather than your precious wedding ring.
Not surprisingly, these abundant metals often behave differently than precious metals. Louie’s group tries to develop catalysts that can completely replace precious metal catalysts. However, sometimes they have orthogonal reactivity. That’s okay too.
“For a synthetic chemist, a builder if you will, that’s actually good, because you want many tools that do totally different things,” Louie said. She describes it as a two-pronged approach: either we’ve made a better hammer that will replace all the really expensive hammers out there, or we’ve made a mallet, and it’s kind of hammer-like, but it’s different.
“It’s a perfect opportunity to find new tools, whether to replace ones that we have or to expand the existing toolbox.”
The foundation of this research was a desire to do something better for the environment. One of Louie’s first projects centered on finding a catalyst that could utilize carbon dioxide as a substrate.
“Carbon dioxide is responsible for all sorts of environmental problems. But if you think from a synthetic point of view, carbon dioxide would be the ideal C1 starting material,” Louie explained. “It’s safe, nontoxic, dirt cheap, and readily available (in fact, it’s a waste product). Maybe we won’t use it on enough scale that we change the amount of carbon dioxide in the atmosphere, but it’s a great resource we can tap into.”
This idea led to the development of a nickel catalyst that could utilize carbon dioxide as a C1 source. As Louie’s group continued to push the catalyst, it turned out that the nickel catalyst could be used to make a wide variety of heterocycles. She still has a project examining the interaction between nickel and carbon dioxide and what it can do, as well as a project using iron as a catalyst to make heterocycles.
The synthetic chemists using Louie’s earth abundant catalysts must be happy that she never swayed from her desire to do fundamental research. She’s adding new tools to the toolbox of chemistry.
