By Bethany Halford
Reprinted from C&EN
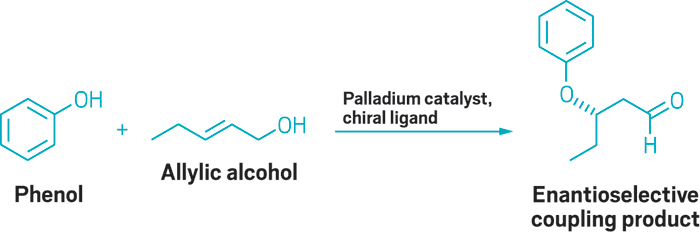
Although chemists have plenty of ways to add carbon atoms to alkenes—including the Nobel Prize-winning Heck reaction—they have fewer options when working with heteroatom nucleophiles, such as alcohols. Now, Matthew S. Sigman and his group at the University of Utah report an intermolecular oxa-Heck reaction that allows them to couple phenols with allylic alcohols in an enantioselective manner (J. Am. Chem. Soc. 2016, DOI: 10.1021/jacs.6b11486). The reaction, which is catalyzed by palladium in the presence of a chiral pyridine oxazoline ligand, creates building blocks that would be tough to make using more common synthetic reactions, such as a Michael addition (example shown). Sigman’s team selected allylic alcohols as the alkene in the reaction in order to influence which hydrogen would be removed during the β-elimination to form the chiral product. The chemists show the reaction works with a broad scope of allylic alcohols and phenols, and even successfully swaps the phenol for cumene hydroperoxide in several examples. Next, they plan to explore the scope of the reaction with other heteroatom nucleophiles that are compatible with the coupling strategy.
- Chemical & Engineering News
- ISSN 0009-2347
- Copyright © 2016 American Chemical Society