 |
| The antibiotic team in Ryan Looper’s lab: Dustin Williams, Travis Haussener, Ryan Looper, Hari Kanna Reddy, Chad Testa, Jack Mohr, & Paul Sebahar |
Development of new antibiotics has been dwindling at a time when we need them most. Antimicrobial resistant infections are estimated to cause 10 million annual deaths around the globe (more than cancer), at an economic cost of $100 trillion, by 2050.
Professor Ryan Looper is working to address this looming crisis. Research in the Looper lab has led to two promising compounds for antibiotic development – a new compound that hits a new site in the ribosome, and an antibiofilm antibiotic called CZ-99.
“These are two concepts that are fundamentally interesting in terms of understanding how the molecules work and what they do,” Looper said. “That alone is something that the antibiotic development community needs, because we’ve seen a complete lack of new directions in this field.”
Biofilms, one focus of Looper’s research, are a leading cause of antibiotic resistance. First, the drug can’t break through the protective biofilm to kill bacteria. Then, the surviving bacteria, closely confined in the biofilm, much more efficiently transfer the developed resistance from bacterium to bacterium. In fact, bacteria in biofilms are up to 1,000 times more resistant to antibiotics.
Research in the past ten years has concentrated on how biofilms are formed. Only recently have scientists started thinking about how to disperse biofilms. According to Looper, nobody has ever developed a drug specifically for biofilms.
“It turns out that is very different than developing a drug for planktonic bacteria.”
Looper began tackling this challenge in collaboration with Dustin Williams, co-director of the Bone and Joint Research Lab in the VA Medical Center and Assistant Professor in the Department of Orthopaedics.
Over time, bacteria in biofilms become resource limited. Eventually they disperse themselves to go feed, and then hunker back down into a biofilm. The literature suggested that as biofilms matured, polyamines become increasingly expressed, and once their concentration gets to a certain point, the biofilm will disperse.
The Looper group identified some specific attributes of the polyamines that help biofilms disperse, and then focused on merging that dispersal with elements of known antibiotics.
“We were looking for compounds that can interact with the protective layer of the biofilm, but also interact with the cell membrane of the bacteria, because there are some chemical correlations between the compositions of those two,” Looper explained. “The idea was to design a molecule that can interact with both, so that you disperse the biofilm and you disperse the bacteria cell walls, killing them. Essentially these become very specific, very selective detergents for bacterial cell membranes and bacterial biofilms.”
’ |
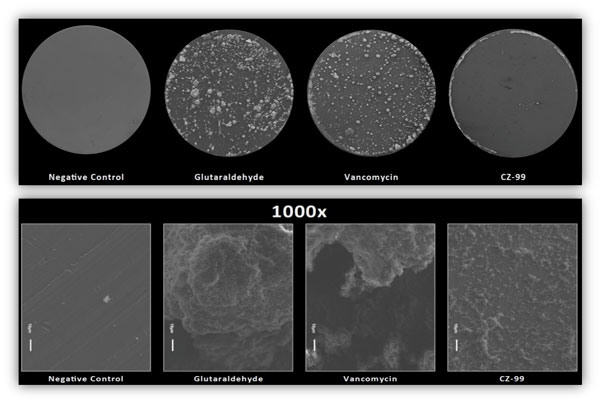
They’re also very effective. CZ compounds disperse and kill MRSA biofilms better than Vancomycin, our “last line of defense” in antibiotics.
The synthesis of this molecule, CZ-99, is a huge accomplishment in and of itself. Now it faces the road to FDA approval and commercialization.
That’s where partner pharmaceutical company Curza Global steps in. Curza will head pre-clinical development and safety studies, leading to an Investigational New Drug (IND) Application and getting CZ-99 into big pharma’s hands. They are a bridge over what Looper calls “the valley of death of academic drug discovery.”
Part of that process is also identifying where CZ-99 also has the most profound use. Biofilms are a problem in paint, in oil pipelines, in chronically infected knee implants, cystic fibrosis infections, diabetic foot ulcers, and tuberculosis. A very promising aspect is its efficacy against Borrelia, the bacterium behind Lyme disease. These spirochetes form biofilms, allowing Lyme disease to persist in low chronic levels of infection over long periods of time. CZ-99 may accelerate the dispersal of those spirochete biofilms.
“The ultimate hope is that these sorts of chronic infections can be helped by this new class of drugs,” Looper said.
Because no other antibiofilm antibiotics currently exist, CZ-99 will first have to be approved as a traditional antibiotic. Ideally, this compound’s approval will help understand biofilm-related infections and how to prove clinical success of treating them. In the future, antibiofilm drugs that have synergy with traditional antibiotics, like CZ-99, may have specific FDA guidelines for approval.
