A data-intensive approach to mechanistic elucidation applied to chiral anion catalysis
By Anat Milo, Andrew J. Neel, F. Dean Toste, Matthew S. Sigman
ABSTRACT: Knowledge of chemical reaction mechanisms can facilitate catalyst optimization, but extracting that knowledge from a complex system is often challenging. Here, we present a data-intensive method for deriving and then predictively applying a mechanistic model of an enantioselective organic reaction. As a validating case study, we selected an intramolecular dehydrogenative C-N coupling reaction, catalyzed by chiral phosphoric acid derivatives, in which catalyst-substrate association involves weak, noncovalent interactions. Little was previously understood regarding the structural origin of enantioselectivity in this system. Catalyst and substrate substituent effects were probed by means of systematic physical organic trend analysis. Plausible interactions between the substrate and catalyst that govern enantioselectivity were identified and supported experimentally, indicating that such an approach can afford an efficient means of leveraging mechanistic insight so as to optimize catalyst design.
Read the full article in Science.
 |
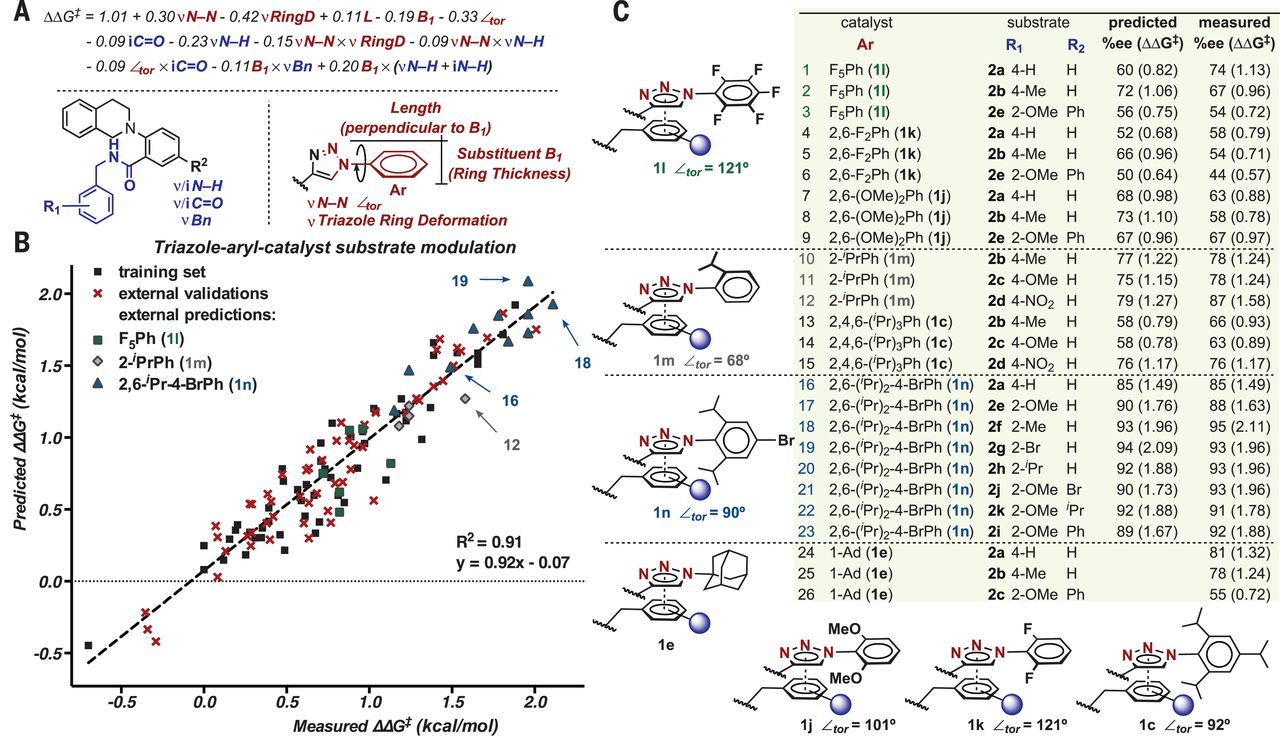
| Fig. 6Validation of mechanistic hypotheses through directed catalyst design.
(A) Normalized equation for the prediction of enantioselectivity by using parameters that describe catalyst and substrate structural features. (B) Predicted versus measured ΔΔG‡ plot. (C) Hypothesis-driven external predictions and their comparison with other relevant catalysts. |
